Logan Bartholomew A personal website, interactive CV, and project repository.
Research
University of California, Berkeley
August 2020 to present (Graduate Research)
Pyrimidine Ring Contraction
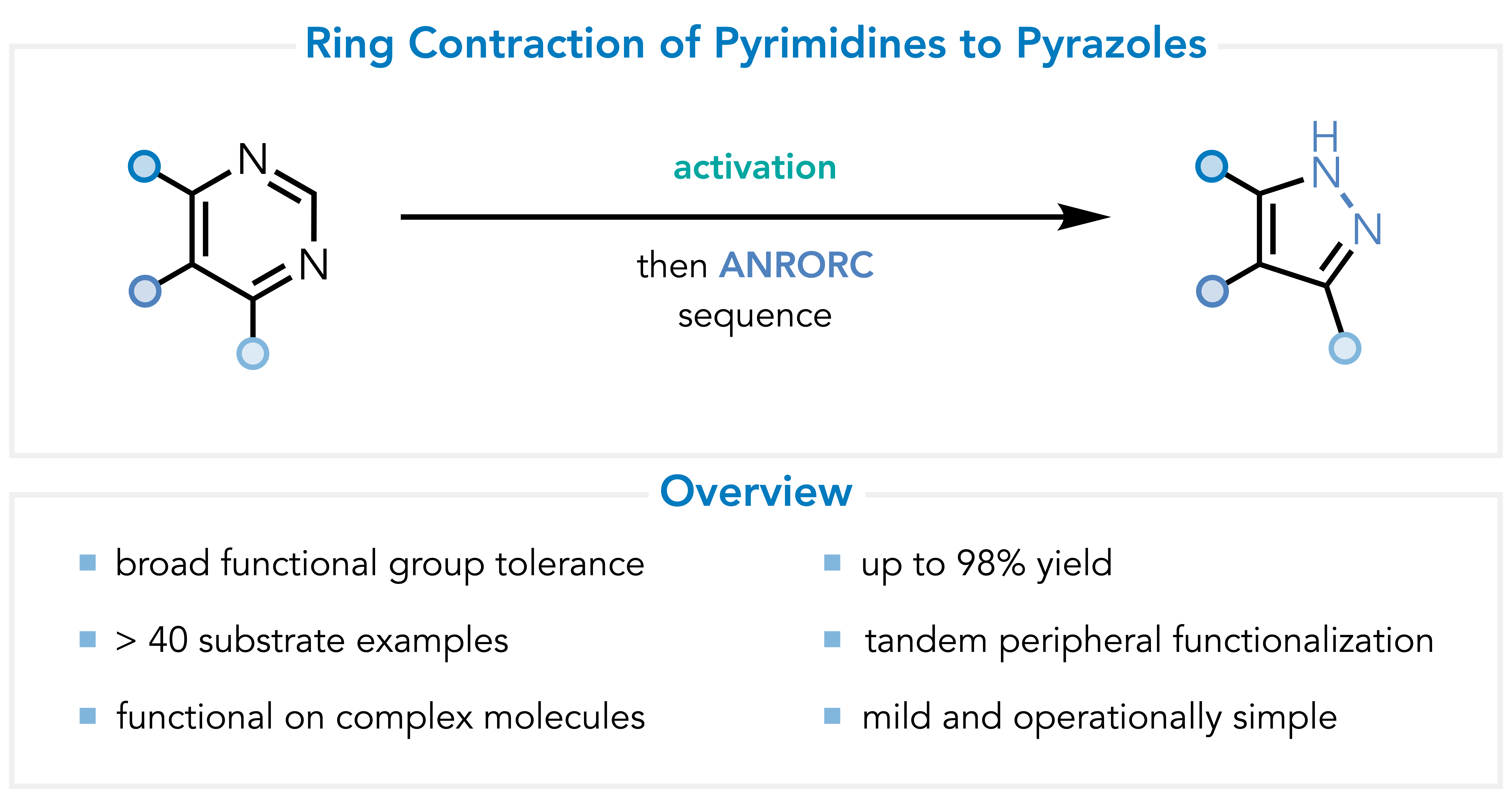
I developed a practical method for pyrimidine ring contraction to pyrazoles through formal carbon deletion, achieving high yield and broad tolerance. This methodology may be used to leverage the favorable directing capabilities and selective functionalizability of the pyrimidine heterocycle for the synthesis, handling, and functionalization of late-stage, pyrazole-containing molecules. Additionally, substituted hydrazines may be used to forge N-functionalized pyrazoles in a regioselective fashion. This work was published in JACS in 2022. A free PDF download is available here.
Azine Isotopic Labeling
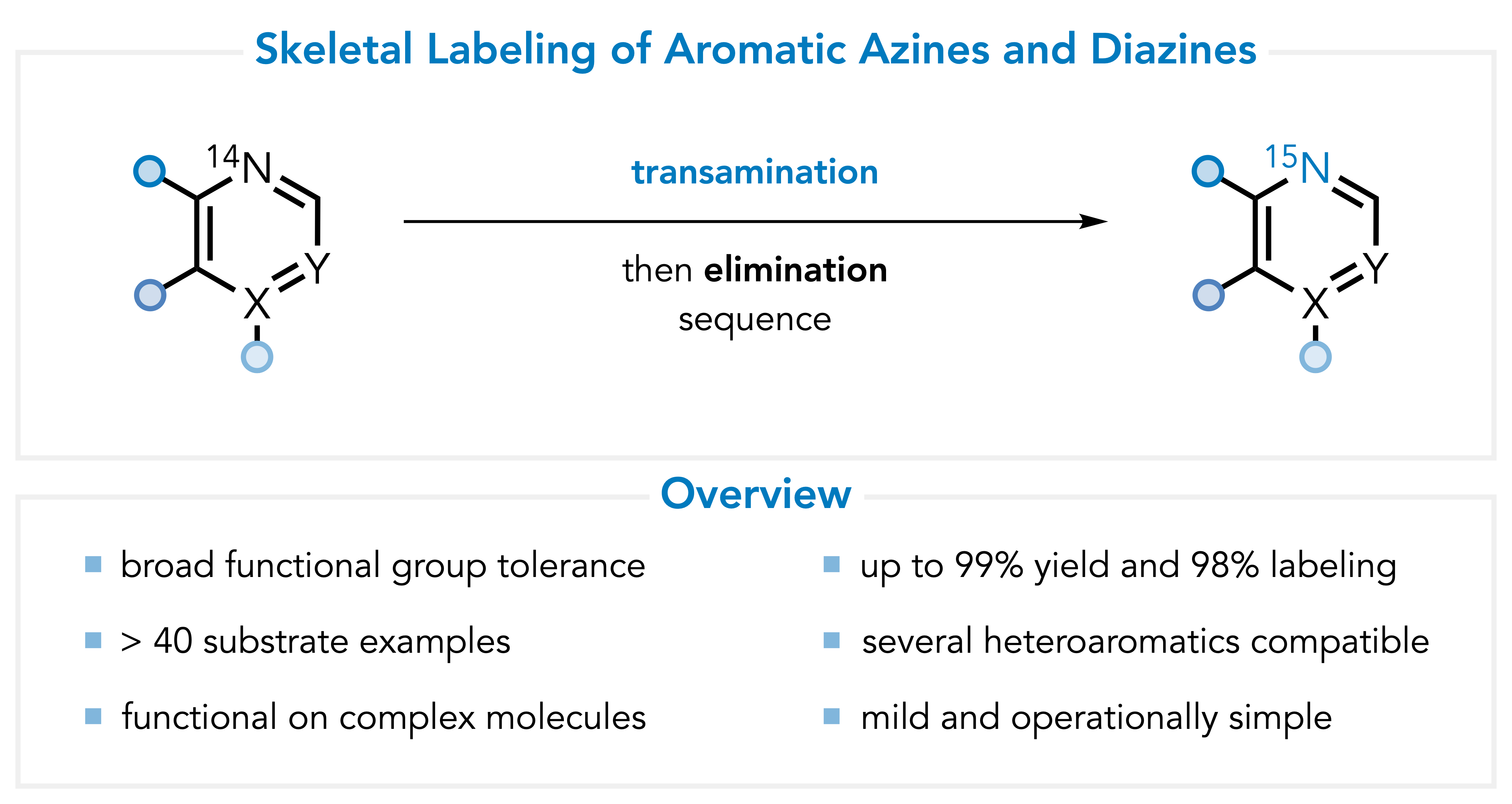
In collabroation with Merck and the Sigman group at the University of Utah, I also developed a mild, direct, one-pot, transaminative methodology for 15N labeling of nitrogen heteroaromatics. I explored the reactivity of azinium and diazinium ylides towards dipolarophiles to prepare polycyclic cycloadducts. This work was published in JACS in 2024. A free PDF download is available here.
Total Synthesis of Haplomintrins
Placeholder
New Halogenation Reagents
I engineered a library of organic halogenating reagents with electrophilic C–halide bonds for enantioselective halofunctionalization in non-coordinating compounds. Critically, all 4 generations of these reagents may be prepared in high yield without chromatography using nontoxic reagents and mild conditions.
Photomediated Azole Transposition
Placeholder
Cheminformatics Projects
I evaluated a programmatic platform for multidirectional reaction prediction with the Grzybowski Group at UNIST-gil, consulted for retrosynthetic prediction software for natural products synthesis with the Coley Group at MIT, and developed cheminformatics scripts for impact analysis with the Paton Group at CSU. I am currently working with the Sigman group to lead the development of a Python-based cheminformatic platform for analyzing the potential impact of theoretical skeletal edits to heteroaromatics.
#### Teaching and Mentorship
I served as a Graduate Student Instructor for CHEM 12A and Lab (Organic Chemistry for Chemistry Majors, Prof. Alanna Schepartz) and as Instructor, Lecturer, and Course Designer for CHEM 115 (Advanced Organic Chemistry, Prof. Dean Toste). I also mentored one junior graduate student, two visiting students, and one undergraduate.
The George Washington University
2016 – 2020 (Undergraduate Research)
I synthesized and assessed a library of >30 fosmidomycin derivatives against Plasmodium falciparum and Mycobacterium tuberculosis with the aim of identifying potential drug candidates for combating malaria and tuberculosis. These molecules were designed to bind to both the active and NADPH cofactor binding sites of the metabolic enzyme Dxr, which is present in both pathogens. Additionally, I developed a new, concise, and modular synthetic route to these analogues. I also prepared several pro-drug variants of some of these compounds. This work is summarized in my Undergraduate Honors Thesis.
Vertex Pharmacueticals, Inc.
2020 (Remote Summer Internship)
Advised by Dr. David Stephens and Dr. Rebecca Swett, I assessed the application of the Buchwald-Hartwig amination in synthesizing a stage 2 Active Pharmaceutical Ingredient (API). Utilizing Quantum Mechanics (QM) methods, I calculated the catalytic cycle’s energy profile, providing insights into the reaction mechanism. I also engaged in the parameterization of ligands for reactivity correlation, employing machine learning for multiple and non-linear regression analysis.
A significant aspect of my work involved the investigation of off-cycle pathways leading to undesired products, utilizing computational tools to understand and mitigate potential challenges in the synthesis process. To facilitate data analysis, I developed Python and Excel packages, which were distributed interdepartmentally for widespread use.
2019 (Summer Internship)
Advised by Dr. Stefanie Roeper, I developed, executed, and optimized a modular, versatile, and efficient synthetic pathway for polysubstituted indoles and indazoles present in a Stage 2 API. I also evaluated the kinetic and thermodynamic profiles of the route, preparing safety and toxicity analyses. This involved close collaboration with chemical engineering teams to successfully complete joint projects.